
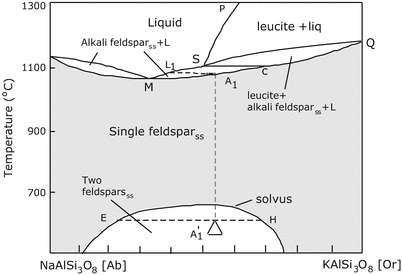
This structure is the building block of many important minerals in the crust and mantle. Introduction This tutorial develops a simple example whose goal is graphical inspection ternary feldspar relationships within the CaO-Na2O-K2O (-Al2O3-SiO2) system. Our goal for this paper is to introduce the ternary diagram and discuss these advantages in hopes that this will stimulate broader use of ternary diagrams and further research into their educational utility. Silicon and oxygen bond covalently to create a silicate tetrahedron (SiO 44- ), which is a four-sided pyramid shape with oxygen at each corner and silicon in the middle (Figure 5.21). This also means that the plot can be used to compare relative rates of energy change during various processes. The left edge of the plot represents the solid solution series of the alkali feldspars. The ternary diagram has some significant advantages over other graphical representations of energy distributions: an entire scenario can appear in a single plot, even when using very small time steps. One of the most familiar examples of a triangular plot in the Earth sciences illustrates the classification of feldspar minerals. Last year the lead author began using ternary diagrams in his introductory (calculus-based) physics course in a novel context-tracking the distribution of energy in a system as it transforms among three categories (e.g., gravitational, kinetic, and thermal) or transfers among three objects (e.g., inductor, capacitor, and resistor). They are familiar to mineralogists (who typically use them to categorize varieties of solid solution minerals such as feldspar) but are not yet widely used in the physics community. A ternary diagram is a graphical representation used for systems with three components.


 0 kommentar(er)
0 kommentar(er)
